Hydration Basics
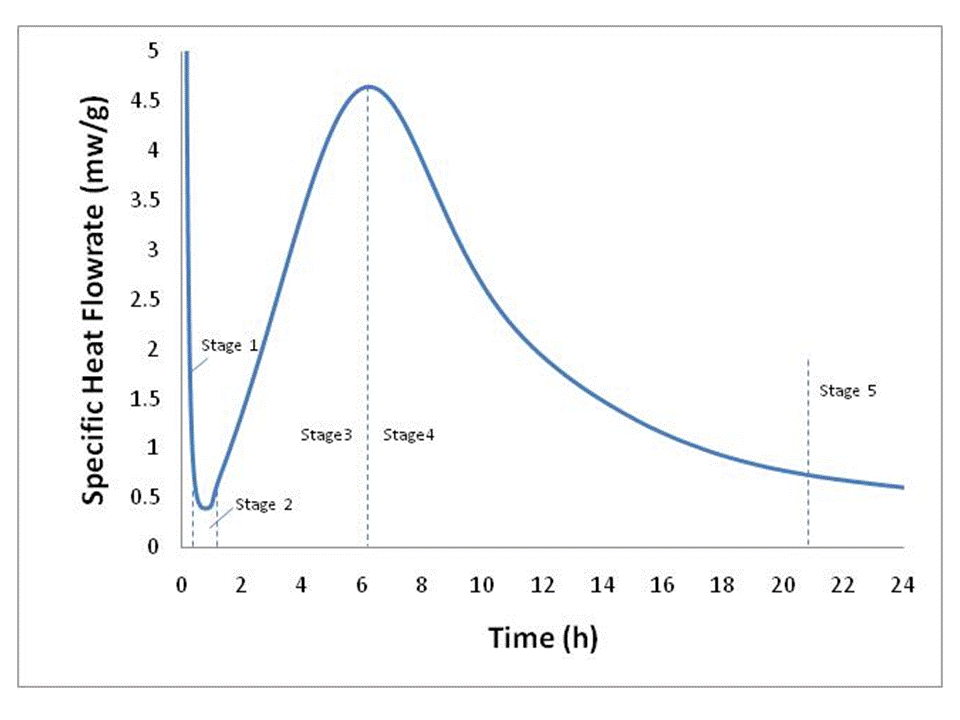
Portland cement (PC) is made up of five major compounds: tricalicum silicate (C3S), dicalcium silicate (C2S), tricalicum aluminate (C3A), tretracalcium alumino ferrite (C4AF) and gypsum (calcium sulfate dihydrate, C$H2). Since C3S is the major constituent of ordinary Type I PC (actually the form of C3S known as alite), it is frequently used as a model in place of the more complex mixture. Alite and likewise portland cement hydration is exothermic, e.g. releases heat, therefore, calorimetry is often times used as a way to study the rate of hydration under various conditions. Figure 1 is a typical isothermal calorimetry curve for alite at 25oC and a w/c (water to cement ratio) of 0.4. It is the shape of this curve that has been the subject of debate and many investigations for decade.
Figure 1. Typical alite calorimetry curve characteristic of C3S-based hydraulic cements, data after Xie and Biernacki [1].
Hydration can be described in six stages: (Stage 0) rapid dissolution lasting on the order of ten minutes or less (not shown); (Stage 1) first deceleration; (Stage 2) induction or dormancy (Stage 3) acceleration; (Stage 4) second deceleration and (Stage 5) slow continued reaction. While these stages have been described many times in the literature and have become the fingerprint for cement hydration, they are the unexpected result of an extremely complex process that is yet to be defined, even for pure cement phases such as C3S or alite.
Cement Shorthand Notation: C=CaO; H=H2O; S=SiO2; $=SO4; A=Al2O3; F=Fe2O3
[1] T. Xie and J. J. Biernacki, The Origins and Evolution of Cement Hydration Models, Comp. Concr., 8(6), 647-675 (2011).